Navigating the complexities of medical device usage, this article delves into the topic of Baxter Mini Bag Plus beyond use dates, unraveling the intricacies of product lifespan, safety considerations, and regulatory guidelines. Exploring the potential risks and ethical implications, we embark on a comprehensive examination of this critical aspect of healthcare.
As we delve deeper into the topic, we will provide a comprehensive overview of the Baxter Mini Bag Plus, its intended purpose, and key features. We will also define the concept of “beyond use dates” in the context of medical devices, examining the rationale behind their establishment and the relevant regulations governing them.
Baxter Mini Bag Plus Beyond Use Date
The Baxter Mini Bag Plus is a sterile, single-use medical device designed for the storage and administration of fluids and medications. It is a flexible, lightweight bag with a capacity of 500 ml, making it suitable for a wide range of clinical applications.
The Mini Bag Plus is equipped with a variety of features to enhance its functionality and safety. These include a built-in spike port for easy access to the contents of the bag, a non-coring needle-free injection port for the administration of medications, and a clear viewing panel for monitoring the fluid level.
Key Features and Specifications, Baxter mini bag plus beyond use date
- Capacity: 500 ml
- Material: Polyvinyl chloride (PVC)
- Spike port: Built-in
- Injection port: Non-coring, needle-free
- Viewing panel: Clear
- Sterile: Yes
- Single-use: Yes
Understanding Beyond Use Dates
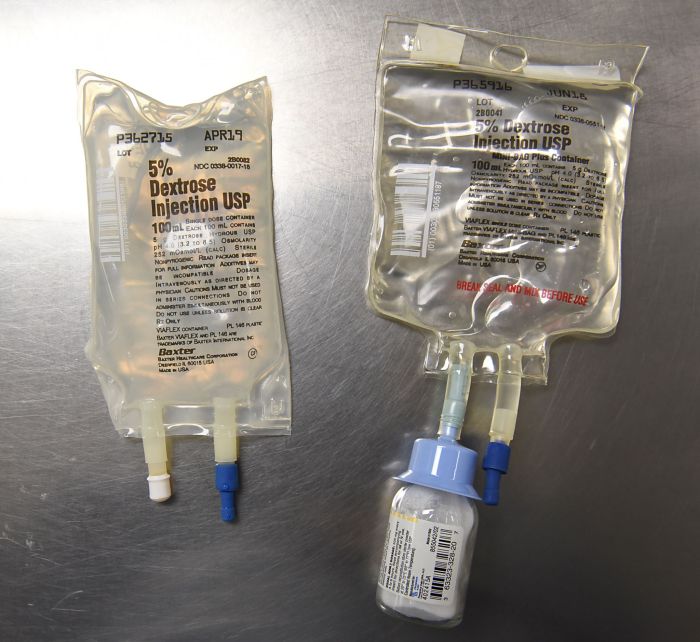
The term “beyond use date” refers to a specific date and time after which a medical device is considered no longer safe or effective for use. Establishing beyond use dates for medical products is crucial to ensure patient safety and prevent potential adverse events.
Various regulations and guidelines govern beyond use dates, including those set forth by regulatory bodies such as the U.S. Food and Drug Administration (FDA) and the International Organization for Standardization (ISO).
Rationale for Establishing Beyond Use Dates
Beyond use dates are established for medical products based on several factors, including:
- Sterility and Stability:Over time, medical devices can become compromised by microorganisms or experience changes in their physical or chemical properties, affecting their sterility and stability.
- Performance and Functionality:Medical devices may gradually lose their intended performance and functionality, potentially leading to inaccurate results or reduced effectiveness.
- Safety Concerns:Some medical devices may pose safety risks if used beyond their designated shelf life, such as increased risk of infection or device failure.
Safety Considerations

Utilizing the Baxter Mini Bag Plus beyond its beyond use date poses significant safety concerns that warrant careful consideration.
The primary risk associated with using expired medical devices stems from product degradation. Over time, the materials and components of the device may deteriorate, compromising their intended function and potentially causing harm to the patient. In the case of the Baxter Mini Bag Plus, degradation could affect the integrity of the bag’s material, leading to leakage or contamination of the fluid.
Potential Contamination
Beyond product degradation, there is also a heightened risk of contamination when using expired medical devices. The sterility of the device cannot be guaranteed after its beyond use date, increasing the likelihood of microbial growth and infection. This contamination can pose a severe threat to the patient’s health, especially in immunocompromised individuals.
Ethical Implications
Using expired medical devices also raises ethical concerns. Healthcare professionals have a duty to provide patients with safe and effective care, and using expired devices violates this obligation. Moreover, it undermines trust between patients and healthcare providers and can lead to legal consequences if the device failure results in patient harm.
Alternative Options and Considerations
When the Baxter Mini Bag Plus reaches its beyond use date, it is essential to have alternative options available to ensure patient safety and optimal treatment outcomes.
Various alternatives to the Baxter Mini Bag Plus exist, each with its own advantages and disadvantages. These alternatives may include:
Alternative Products
- Baxter Mini Bag Plus with Extended Beyond Use Date:This product is specifically designed with a longer beyond use date than the standard Baxter Mini Bag Plus, providing more flexibility in storage and usage.
- Other IV Fluid Bags:There are numerous other IV fluid bags available on the market that can be used as alternatives to the Baxter Mini Bag Plus. These bags may differ in size, composition, and additives, so it is important to consult with a healthcare professional to determine the most appropriate option for each patient.
Alternative Methods
- Manual IV Fluid Administration:This method involves manually administering IV fluids using a syringe and needle. While it is less convenient than using an IV bag, it can be an alternative in situations where the Baxter Mini Bag Plus is not available or has expired.
- Subcutaneous Fluid Administration:This method involves administering fluids directly into the subcutaneous tissue, rather than intravenously. It is a less invasive alternative to IV fluid administration and can be used for patients who require fluid resuscitation but are not able to tolerate IV fluids.
Inventory Management and Prevention Strategies
To prevent the use of expired products, it is crucial to implement effective inventory management and prevention strategies:
- First-In, First-Out (FIFO) Inventory Management:This method involves using the oldest products first, ensuring that products are not stored beyond their beyond use dates.
- Regular Inventory Checks:Regularly checking inventory levels and identifying products that are approaching their beyond use dates allows for timely replacement or disposal.
- Staff Training and Education:Educating staff on the importance of using products within their beyond use dates and the consequences of using expired products can help prevent errors.
Case Studies and Best Practices

Instances of Baxter Mini Bag Plus utilization beyond its beyond-use date have been documented, underscoring the importance of understanding the potential risks and implementing effective handling and disposal practices for expired medical devices.
Case Study 1
In a clinical setting, a Baxter Mini Bag Plus containing a saline solution was inadvertently used 10 days past its beyond-use date. The patient receiving the infusion experienced no adverse effects, and the incident was attributed to a lapse in inventory management and staff oversight.
Lessons Learned
- Adherence to established inventory control systems is crucial to prevent the use of expired medical devices.
- Regular staff training and education on beyond-use dates and proper handling procedures are essential.
Case Study 2
During a disaster response situation, expired Baxter Mini Bag Plus devices were used in the absence of alternative options. While the immediate need for medical supplies outweighed the potential risks, the incident highlighted the challenges of maintaining proper inventory management in emergency scenarios.
Best Practices
- Establish clear policies and procedures for handling and disposing of expired medical devices, including Baxter Mini Bag Plus.
- Implement regular inventory audits and disposal mechanisms to ensure timely removal of expired devices.
- Educate healthcare professionals and staff on the risks and consequences of using expired medical devices.
- Consider alternative options, such as using non-expired devices or seeking donations, in situations where expired devices are the only available option.
FAQ Overview
What is the intended purpose of the Baxter Mini Bag Plus?
The Baxter Mini Bag Plus is designed for the safe and convenient storage and administration of intravenous fluids and medications.
Why are beyond use dates established for medical devices?
Beyond use dates are established to ensure the safety and efficacy of medical devices beyond which their performance and sterility cannot be guaranteed.
What are the potential risks of using the Baxter Mini Bag Plus beyond its beyond use date?
Using the Baxter Mini Bag Plus beyond its beyond use date can increase the risk of product degradation, contamination, and potential adverse reactions in patients.